多囊卵巢综合征(polycystic ovary syndrome,PCOS)是一种生殖功能障碍与糖脂代谢异常并存的生殖内分泌紊乱综合征。PCOS的临床特征表现呈高度异质性和复杂性,其症状几乎伴随女性的一生[1]。流行病学研究显示PCOS影响约5%~18%的育龄期女性和3%~11%的青少年[2]。PCOS的发病机制目前尚不明确,现有研究集中关注PCOS患者卵巢颗粒细胞(granulosa cell)的异常凋亡,认为异常凋亡会造成颗粒细胞数量急剧下降,卵泡营养障碍而生长受限,进而导致患者卵泡闭锁及排卵功能障碍[3]。同时学界证实通过改变颗粒细胞异常凋亡的病理状态可有效缓解PCOS的临床症状,甚至妊娠结局。目前发现胰岛素抵抗(insulin resistance,IR)、高雄激素血症(hyperandrogenism,HA)、氧化应激、慢性低度炎症和脂质代谢异常均与颗粒细胞凋亡关系密切[4]。中医古籍中并无PCOS的病名,诸多医家认为窠囊理论是揭示PCOS中医发病机制起源最久、研究最深入的理论。朱丹溪的“自气成积,自积成痰,痰夹瘀血,遂成窠囊”和沈金鳌的“七日食痰,饮食不消,或夹瘀血,遂成窠囊”高度概括了PCOS的形成与痰浊、气滞、血瘀等病理因素密切相关。肾为天癸之源,肾虚为窠囊的病理基础。PCOS病机为虚实夹杂,本虚标实,本虚以肾虚为关键,涉及痰浊、气滞、血瘀等病理因素破坏肾-天癸-冲任-胞宫轴功能。PCOS致病机制复杂,中医药从整体观、个体化特色出发,在干预卵巢颗粒细胞凋亡治疗PCOS方面有巨大优势,这在临床和动物实验上已经得到验证。本文系统梳理中医药调控PCOS患者卵巢颗粒细胞凋亡的作用机制,为临床防治PCOS以及新药研发提供科学客观的新视角。
1 颗粒细胞凋亡概述
细胞凋亡是机体内细胞主动的、可控的、程序性死亡方式,涉及一系列复杂的生化特征改变,最终使细胞从体内清除,维持机体内环境稳态。在女性一生中,细胞凋亡是卵巢生理学的典型事件,其中包含卵泡闭锁、黄体萎缩、月经排出等。颗粒细胞是卵泡中包裹在卵母细胞周围的扁平或梭形细胞,其正常增殖是维持卵巢基本功能的应答表现,颗粒细胞作为卵巢的基础功能单位,其凋亡在优势卵泡的选择中发挥重要作用。PCOS引起的卵泡生长紊乱主要有两个异常:一是窦前卵泡发育加速,卵泡程序化发育发生改变;二是窦状卵泡生长障碍及卵泡发育停滞。虽然PCOS患者窦卵泡发育停滞的机制尚未彻底阐明,但现有研究认为在下丘脑-垂体-卵巢轴的调控下,卵泡生长发育这一具有严格时限性的动态过程主要受卵巢局部微环境的调节,即卵巢分泌的多种生长因子及颗粒细胞凋亡的影响。研究表明,当颗粒细胞生存的外部环境受到PCOS相关复杂机制(如IR、HA、氧化应激、慢性低度炎症和脂质代谢异常等)刺激时,B细胞淋巴瘤-2(B-cell lymphoma-2,Bcl-2)家族中抗凋亡成员与促凋亡成员比例失调,触发细胞凋亡信号,胱天蛋白酶(Caspase)家族从非活性状态转化为具有活性的功能蛋白酶。PCOS最终通过调控Caspase家族的表达,经线粒体途径介导多种促凋亡蛋白质释放,形成凋亡小体,进而级联激活Caspase家族,促进颗粒细胞凋亡。
2 PCOS相关因素与卵巢颗粒细胞
2.1 IR与卵巢颗粒细胞
IR是机体生理水平的胰岛素产生的生物学效应低于预期水平,葡萄糖摄取和利用胰岛素的效能下降,是组织内胰岛素调节糖代谢处于代偿阶段的标志。IR是PCOS发生发展的主要病理改变之一,既往研究已证实IR影响颗粒细胞的生长发育,进而影响卵泡的生长和成熟[5]。卵巢颗粒细胞中磷脂酰肌醇3激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase B,Akt)通路是胰岛素在组织中发挥作用的经典途径,药物治疗可增加PI3K/Akt的磷酸化水平,显著改善细胞对胰岛素的敏感性,加速能量代谢,进而促进颗粒细胞生长,维持卵泡发育[6]。同时,富集在PI3K/Akt信号通路中的微小RNA靶基因转录后调控也可介导卵巢局部IR[5]。PCOS患者体内IR的发生与丝裂原激活的蛋白激酶(mitogen-activated protein kinase,MAPK)/细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)通路异常活化相关,通过酶促级联反应实现胰岛素信号从胞外到胞内细胞核的传递,调控以IR为核心的卵巢微环境[7]。
2.2 HA与卵巢颗粒细胞
HA在PCOS发病机制研究中处于关键地位,学界也存在将PCOS视为卵巢源性HA的学术观点。既往研究已证实,产前雄激素暴露会导致子代PCOS发生率增加[8]。目前研究表明高雄激素通过上调PCOS大鼠颗粒细胞的动力相关蛋白1(dynamin-related protein1,Drp1)水平破坏线粒体动态平衡,使其呈现颗粒状结构,或通过改变线粒体膜电位促进颗粒细胞凋亡与卵泡中热休克蛋白(heat shock protein,HSP)表达降低,减弱HSP的细胞保护作用,致Bcl-2相关X蛋白(Bcl-2 associated X protein,Bax)/Bcl-2上调,激活Caspase级联反应,导致颗粒细胞凋亡[9]。最新的研究关注高雄激素环境下颗粒细胞生物学功能的改变,通过比较体外高雄激素与非高雄激素环境下小鼠颗粒细胞的转录组信息,揭示了氧化应激与炎症在高雄激素引发的PCOS小鼠颗粒细胞功能损伤中的关键作用[10]。
2.3 氧化应激与卵巢颗粒细胞
氧化应激是指氧化-抗氧化系统失衡,产生过量的活性氧(reactive oxygen species,ROS),进而引起细胞和组织发生系列生理病理反应。氧化应激被认为是细胞再生系统中凋亡的关键因素。氧化应激可通过外源性途径、内源性途径和内质网应激等多种途径诱导卵巢颗粒细胞凋亡,导致卵巢细胞营养剥夺,使卵巢微环境代谢紊乱,从而加重卵巢损伤[11]。颗粒细胞增殖需要充足的蛋白质、碳水化合物和脂质供应。然而,氧化应激可直接破坏生物大分子结构,导致颗粒细胞凋亡、黄体功能障碍等,从而加速卵巢衰老[12]。研究显示,PCOS患者卵泡液中过量脂肪酸堆积[13]及颗粒细胞糖酵解功能受到抑制[14]等因素均可导致机体产生过量的ROS,通过线粒体裂解途径导致线粒体功能障碍,触发Caspase级联反应,促使颗粒细胞凋亡。因此,适当改善氧化应激状态对PCOS患者具有正向调节作用。
2.4 慢性低度炎症与卵巢颗粒细胞
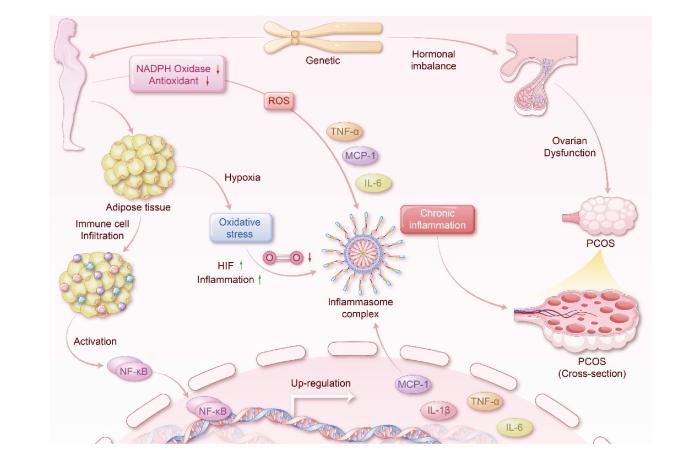
继Kelly等[15]报道PCOS患者可能存在慢性低度炎症以来,“炎症学说”逐渐成为学界深入探索PCOS发病机制的研究热点。慢性低度炎症是由营养物代谢过剩蓄积诱导的炎症反应,属感染性和自身免疫性炎症水平以下的炎症。在外周血、卵泡液、卵巢组织、子宫内膜细胞及颗粒细胞中均可检测到炎症因子的异常表达,慢性低度炎症作为枢纽通常与肥胖、IR、氧化应激、HA之间相互作用。在脂肪组织中,经典活化的M1型巨噬细胞与M2型巨噬细胞稳态失衡会导致炎症因子的产生和释放。促炎因子肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、单核细胞趋化蛋白-1(monocyte chemotactic protein-1,MCP-1)、白细胞介素-6(interleukin-6,IL-6)在肥胖女性体内高表达,提示肥胖个体的促炎症过程占据主导地位,促炎因子通过破坏PCOS女性体内的ROS自由基平衡促使胰岛β细胞凋亡,或激活核因子κB(nuclear factor-κB,NF-κB)信号通路,阻碍胰岛素信号通路,引起IR,影响糖脂代谢并加重全身炎症状态。此外,遗传及生活方式可促使激素失调,PCOS女性内脏脂肪组织蓄积,体内的氧化应激水平升高,激活NF-κB信号通路,致使炎症细胞募集。参与PCOS发生发展的炎症介质和相关因素如图1所示。
图1
图1
慢性低度炎症与PCOS的关系
注:NADPH Oxidase NADPH氧化酶,Antioxidant 抗氧化剂,Adipose tissue 脂肪组织,Immune cell Infiltration 免疫细胞浸润,Activation 激活,Hypoxia 缺氧,HIF 低氧诱导因子,Inflammation 炎症,Genetic 遗传,Hormonal imbalance 激素失调,Ovarian Dysfunction 卵巢功能障碍,Chronic inflammation 慢性炎症,Inflammasome complex 炎症复合体,Up-regulation 上调,Cross-section 横截面。
2.5 脂质代谢异常与卵巢颗粒细胞
瘦素(leptin)是下丘脑-垂体-性腺轴的调节激素,参与中枢和外周水平生殖轴的启动调节。瘦素只有在狭窄的浓度范围内才能促进卵泡成熟,瘦素抵抗或瘦素缺乏的肥胖人群多存在瘦素途径的慢性代谢失调,致使卵母细胞质量受损、早期胚胎发育不良以及颗粒细胞功能障碍[16]。趋化素(chemerin)作为新型脂肪因子,其受体可通过激活NF-κB、AMP活化的蛋白质激酶(AMP-activated protein kinase,AMPK)抑制Akt途径,抑制肥胖小鼠颗粒细胞活性,导致颗粒细胞中ROS蓄积和细胞凋亡。趋化素及其受体上调可能解释了肥胖小鼠卵巢氧化应激和凋亡的增加[17]。脂肪间充质干细胞外泌体通过激活胰岛素受体底物1(insulin receptor substrate 1,IRS1)/Akt通路干预细胞葡萄糖稳态,改善PCOS糖代谢异常,进而提高PCOS大鼠生育率及改善卵巢多囊样结构[18]。由此可见,脂肪因子及干细胞外泌体通过调节凋亡关键基因或关键通路干预氧化应激及糖代谢障碍,调控PCOS卵巢颗粒细胞凋亡。
3 中医药干预卵巢颗粒细胞凋亡治疗PCOS
3.1 多酚类
白藜芦醇(resveratrol)是从虎杖根部提取的天然多酚类化合物。Liang等[19]研究发现白藜芦醇具有改变人卵巢颗粒细胞氧化还原的双相反应,高浓度的白藜芦醇可促进氧化,抑制DNA合成,诱导颗粒细胞凋亡,而低浓度的白藜芦醇则显著抑制颗粒细胞凋亡,故临床选用白藜芦醇作为补充剂改善卵巢状态和辅助生殖技术成功率的口服最佳剂量是现今研究的关键。姜黄素(curcumin)是从姜黄根茎中提取的脂溶性多酚。Zhang等[20]研究发现,姜黄素可通过抑制肌醇需求酶1α(inositol-requiring enzyme 1α,IRE1α)/X-框结合蛋白1(X-box binding protein 1,XBP1)水平、激活PI3K/Akt信号通路保护PCOS大鼠卵巢颗粒细胞免受高雄激素诱导的凋亡,姜黄素还能通过纠正体内性激素紊乱实现生理性雄激素平衡,这些效应可协同干预PCOS-HA大鼠状态。张韵函等[21]研究显示与PCOS模型组相比,姜黄素治疗组血清性激素水平和空腹血糖水平得到显著改善,TNF-α、IL-6、C反应蛋白(C-reactive protein,CRP)显著降低,同时,卵泡和卵巢颗粒细胞层中TNF-α及IL-6表达水平显著降低。上述研究提示姜黄素的多靶点作用,如抑制细胞凋亡、纠正体内激素紊乱、调节炎性因子水平等,使其在对PCOS合并HA、慢性低度炎症的治疗中发挥重要作用。
3.2 黄酮类
柚皮素(naringenin)是陈皮、枳实的主要黄酮类活性成分。吕向阳等[22]研究发现,柚皮素通过抑制受体相互作用蛋白1(receptor-interacting protein 1,RIP1)/RIP3/混合谱系激酶结构域样蛋白(mixed lineage kinase domain-like protein,MLKL)凋亡途径活化显著改善PCOS大鼠卵巢颗粒细胞的病理性凋亡。黄芩苷(baicalin)是从安胎圣药黄芩根部提取分离的黄酮类化合物,具有生物活性好和不良反应少的特性。范宏芳等[23]研究发现,PCOS大鼠颗粒细胞凋亡率升高,用黄芩苷处理后可显著降低细胞凋亡率、上调大鼠血清中卵泡刺激素(follicle-stimulating hormone,FSH)、雌二醇(estradiol,E2)、p-PI3K、p-Akt蛋白表达水平,下调睾酮(testosterone,T)和黄体生成素(luteinizing hormone,LH)水平,提示黄芩苷通过激活PI3K/Akt信号通路诱导卵巢颗粒细胞增殖,抑制凋亡,进而改善性激素代谢紊乱及卵巢组织病理损伤。淫羊藿苷(icariin)是从补肾益精药淫羊藿中提取的一种黄酮类化合物。徐琼芳等[24]研究显示,淫羊藿苷呈剂量依赖性地抑制PCOS大鼠卵巢颗粒细胞中基质细胞衍生因子1(stromal cell-derived factor-1,SDF-1)、C-X-C型趋化因子受体4(C-X-C motif chemokine receptor 4,CXCR4)蛋白表达,且SDF-1激活剂减弱了高剂量组淫羊藿苷对颗粒细胞凋亡的抑制作用,验证了淫羊藿苷可能通过抑制SDF-1/CXCR4信号通路干预PCOS大鼠卵巢颗粒细胞凋亡。槲皮素(quercetin)是生物学活性较高且药理作用丰富的黄酮类化合物。江雪娟等[25]研究发现,运用槲皮素及晚期糖基化终末产物(advanced glycosylation end products,AGE)/晚期糖基化终末产物受体(receptor of advanced glycosylation end products,RAGE)通路小分子阻断剂处理卵巢颗粒细胞后,高迁移率族蛋白1(high mobility group box 1,HMGB1)、AGE、RAGE的蛋白表达水平均显著下调,而p-ERK和p-p38 MAPK的蛋白表达水平显著上调,提示槲皮素抑制PCOS大鼠卵巢颗粒细胞凋亡是通过阻断AGE/RAGE通路活性实现的。另外,4-氯-N-环己基-N-(苯基甲基)苯甲酰胺(N-benzyl-4-chloro-N-cyclohexylbenzamide,FZ)阻断剂联合高剂量槲皮素处理卵巢颗粒细胞后,可见颗粒细胞中ROS的荧光强度减弱,提示ROS活性降低。故该研究证实中药成分槲皮素可以增强细胞对氧化应激的抵御能力,减少ROS蓄积,从而减少颗粒细胞凋亡。
3.3 生物碱类
益母草碱(leonurine)是从益母草中提取的生物碱类化合物。尹和芳等[26]研究发现,经益母草碱处理的PCOS大鼠卵巢颗粒细胞的凋亡率、Caspase-3、Ras同源基因家族成员A(Ras homolog gene family member A,RhoA)、Rho相关卷曲螺旋形成蛋白激酶1(Rho-associated coiled coil forming protein kinase,ROCK1)、ROCK2蛋白表达显著降低,Bcl-2蛋白表达显著增加,且呈浓度依赖性,提示益母草碱通过抑制RhoA/ROCK信号通路活化逆转PCOS大鼠的颗粒细胞凋亡。Kuang等[27]研究发现,小檗碱(berberine)可以抑制PCOS患者颗粒细胞中IL-17A、IL-6水平,增加AMPK mRNA和IRS mRNA水平,降低哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)mRNA水平,调节葡萄糖代谢,改善IR,从而达到治疗PCOS的目的。提示小檗碱调节以IR为核心的PCOS患者糖脂代谢的机制不是刺激其胰岛细胞分泌胰岛素,而是增加细胞的葡萄糖消耗,改善糖耐量,达到治疗效果。
3.4 皂苷类
黄芪甲苷(astragaloside Ⅳ)是源于黄芪的高纯度皂苷类化合物。刘冷等[28]通过灌胃来曲唑(letrozole)联合高脂高糖饮食法建立肥胖型PCOS-IR动物模型,相较于模型组,黄芪甲苷治疗组改善了肥胖PCOS-IR大鼠囊肿性卵泡数量增多、颗粒细胞层变薄等多囊样卵巢形态特征,并降低了卵巢组织血管内皮生长因子(vascular endothelial growth factor,VEGF)、p-Raf/Raf、p-MEK/MEK、p-ERK/ERK蛋白表达,表明黄芪甲苷通过抑制MAPK/ERK通路活化拮抗肥胖PCOS大鼠的IR,改善其卵巢多囊样形态,丰富了对黄芪甲苷的药理作用机制研究。
3.5 联苄类
毛兰素(erianin)是从铁皮石斛或鼓槌石斛中提纯分离的联苄类化合物。房丽娜等[29]的研究证实,毛兰素在体外以剂量依赖性方式促进卵巢颗粒细胞增殖并调节性激素水平,其机制是通过减少卵巢组织中凋亡相关蛋白Bcl-2、Bax、Caspase-3的表达使大鼠卵巢组织中大肿瘤抑制因子1(large tumor suppressor 1,LATS1)、Yes相关蛋白(Yes-associated protein,YAP)磷酸化水平减弱,同时上调LATS1、YAP、转录共激活因子(transcriptional aid activating protein,TAZ)蛋白表达水平,抑制Hippo/YAP信号通路激活,促进卵巢颗粒细胞增殖。
4 结语与展望
中医认为PCOS以肾虚为本,痰湿、血瘀、气郁为标,属虚实夹杂证,病机复杂,症状多样,主要采用辨证论治、中医序贯疗法等,同时注重调畅情志、改善体质。中医基础理论与现代凋亡理论也存在相映之处。在机制特点上,基于“阳化气,阴成形”的阴阳气化学说,根据中医取类比象思维对细胞凋亡进行了阐释,总结了中药单体通过经典或非经典通路调控卵巢颗粒细胞凋亡干预PCOS的作用机制。
然而,中医药治疗PCOS的研究尚有一定局限性。中医药基于信号通路干预PCOS卵巢颗粒细胞的机制研究所纳入的动物模型鲜少有明确的中医辨证分型报道,对于构建中医证型的实验研究缺乏预实验模型的筛选,进而会影响实验数据的准确性和客观性。基础研究主要集中在动物和细胞实验研究,缺乏大样本、多中心、高质量的临床试验,导致临床数据参考价值不高。对于PCOS疾病的研究主要集中在生殖系统病变部位,缺乏对下丘脑、神经、血管、肾脏、脂肪等的研究,研究内容也多停留在IR层面。中药单体的信号通路研究多集中于已知经典的PI3K/Akt、AMPK/mTOR信号通路,对于非经典通路有待深入挖掘。因此,今后需依据多样化的辨证体系构建病证结合动物模型,提升中医药精准治疗的认知;围绕多中心开展高质量的临床试验研究,丰富循证内涵;拓宽研究内容,不局限于单一的生殖系统病变部位,深入探索非经典信号通路的机制层面关联性,研究填补PCOS信号通路交互机制的空白。
参考文献
基于年龄-时期-队列模型分析1990—2019年中国妇科疾病负担趋势
[J].
Prevalence and diagnosis of polycystic ovary syndrome (PCOS) in adolescents - what′s new in 2023? Systematic review
[J].
PDE4 inhibitor Roflumilast modulates inflammation and lipid accumulation in PCOS mice to improve ovarian function and reduce DHEA-induced granulosa cell apoptosis in vitro
[J].
多囊卵巢综合征发病机制研究进展
[J].
Trends in insulin resistance: insights into mechanisms and therapeutic strategy
[J].
LncRNA MALAT1对PCOS颗粒细胞凋亡、自噬和PI3K/Akt/mTOR通路的影响
[J].
Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome
[J].What is the recommended assessment and management of those with polycystic ovary syndrome (PCOS), based on the best available evidence, clinical expertise, and consumer preference?International evidence-based guidelines address prioritized questions and outcomes and include 254 recommendations and practice points, to promote consistent, evidence-based care and improve the experience and health outcomes in PCOS.The 2018 International PCOS Guideline was independently evaluated as high quality and integrated multidisciplinary and consumer perspectives from six continents; it is now used in 196 countries and is widely cited. It was based on best available, but generally very low to low quality, evidence. It applied robust methodological processes and addressed shared priorities. The guideline transitioned from consensus based to evidence-based diagnostic criteria and enhanced accuracy of diagnosis, whilst promoting consistency of care. However, diagnosis is still delayed, the needs of those with PCOS are not being adequately met, evidence quality was low and evidence-practice gaps persist.The 2023 International Evidence-based Guideline update reengaged the 2018 network across professional societies and consumer organizations with multidisciplinary experts and women with PCOS directly involved at all stages. Extensive evidence synthesis was completed. Appraisal of Guidelines for Research and Evaluation-II (AGREEII)-compliant processes were followed. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was applied across evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength and diversity and inclusion were considered throughout.This summary should be read in conjunction with the full Guideline for detailed participants and methods. Governance included a six-continent international advisory and management committee, five guideline development groups, and paediatric, consumer, and translation committees. Extensive consumer engagement and guideline experts informed the update scope and priorities. Engaged international society-nominated panels included paediatrics, endocrinology, gynaecology, primary care, reproductive endocrinology, obstetrics, psychiatry, psychology, dietetics, exercise physiology, obesity care, public health and other experts, alongside consumers, project management, evidence synthesis, statisticians and translation experts. Thirty-nine professional and consumer organizations covering 71 countries engaged in the process. Twenty meetings and five face-to-face forums over 12 months addressed 58 prioritized clinical questions involving 52 systematic and 3 narrative reviews. Evidence-based recommendations were developed and approved via consensus across five guideline panels, modified based on international feedback and peer review, independently reviewed for methodological rigour, and approved by the Australian Government National Health and Medical Research Council (NHMRC).The evidence in the assessment and management of PCOS has generally improved in the past five years, but remains of low to moderate quality. The technical evidence report and analyses (∼6000 pages) underpins 77 evidence-based and 54 consensus recommendations, with 123 practice points. Key updates include: i) further refinement of individual diagnostic criteria, a simplified diagnostic algorithm and inclusion of anti-Müllerian hormone (AMH) levels as an alternative to ultrasound in adults only; ii) strengthening recognition of broader features of PCOS including metabolic risk factors, cardiovascular disease, sleep apnea, very high prevalence of psychological features, and high risk status for adverse outcomes during pregnancy; iii) emphasizing the poorly recognized, diverse burden of disease and the need for greater healthcare professional education, evidence-based patient information, improved models of care and shared decision making to improve patient experience, alongside greater research; iv) maintained emphasis on healthy lifestyle, emotional wellbeing and quality of life, with awareness and consideration of weight stigma; and v) emphasizing evidence-based medical therapy and cheaper and safer fertility management.Overall, recommendations are strengthened and evidence is improved, but remain generally low to moderate quality. Significantly greater research is now needed in this neglected, yet common condition. Regional health system variation was considered and acknowledged, with a further process for guideline and translation resource adaptation provided.The 2023 International Guideline for the Assessment and Management of PCOS provides clinicians and patients with clear advice on best practice, based on the best available evidence, expert multidisciplinary input and consumer preferences. Research recommendations have been generated and a comprehensive multifaceted dissemination and translation programme supports the Guideline with an integrated evaluation program.This effort was primarily funded by the Australian Government via the National Health Medical Research Council (NHMRC) (APP1171592), supported by a partnership with American Society for Reproductive Medicine, Endocrine Society, European Society for Human Reproduction and Embryology, and the Society for Endocrinology. The Commonwealth Government of Australia also supported Guideline translation through the Medical Research Future Fund (MRFCRI000266). HJT and AM are funded by NHMRC fellowships. JT is funded by a Royal Australasian College of Physicians (RACP) fellowship. Guideline development group members were volunteers. Travel expenses were covered by the sponsoring organizations. Disclosures of interest were strictly managed according to NHMRC policy and are available with the full guideline, technical evidence report, peer review and responses (www.monash.edu/medicine/mchri/pcos). Of named authors HJT, CTT, AD, LM, LR, JBoyle, AM have no conflicts of interest to declare. JL declares grant from Ferring and Merck; consulting fees from Ferring and Titus Health Care; speaker's fees from Ferring; unpaid consultancy for Ferring, Roche Diagnostics and Ansh Labs; and sits on advisory boards for Ferring, Roche Diagnostics, Ansh Labs, and Gedeon Richter. TP declares a grant from Roche; consulting fees from Gedeon Richter and Organon; speaker's fees from Gedeon Richter and Exeltis; travel support from Gedeon Richter and Exeltis; unpaid consultancy for Roche Diagnostics; and sits on advisory boards for Roche Diagnostics. MC declares travels support from Merck; and sits on an advisory board for Merck. JBoivin declares grants from Merck Serono Ltd.; consulting fees from Ferring B.V; speaker's fees from Ferring Arzneimittell GmbH; travel support from Organon; and sits on an advisory board for the Office of Health Economics. RJN has received speaker's fees from Merck and sits on an advisory board for Ferring. AJoham has received speaker's fees from Novo Nordisk and Boehringer Ingelheim. The guideline was peer reviewed by special interest groups across our 39 partner and collaborating organizations, was independently methodologically assessed against AGREEII criteria and was approved by all members of the guideline development groups and by the NHMRC.Copyright © 2023. Published by Elsevier Inc.
Ovarian expression of follicle stimulating hormone and activin receptors genes in a prenatally-androgenized rat model of polycystic ovary syndrome in adulthood
[J].The expression of genes involved in basic pathways, such as folliculogenesis and steroidogenesis may be affected following prenatal androgen exposure. Besides, exposure to androgens during prenatal life plays a central role in developing polycystic ovary syndrome (PCOS) in females in later life. In the present study, we aimed to examine the expression of the follicle stimulating hormone receptor (FSHR) and activin receptor (actR) genes in ovarian granulosa cells (GCs) of a prenatally-androgenized rat model of PCOS in adulthood.In the adult rat model of PCOS and their controls (n = 8 in each group), different phases of the estrous cycle were determined by vaginal smear. Total RNA was extracted from the ovarian GCs using the TRIzol protocol, a reverse transcription kit was used for complementary DNA (cDNA) synthesis, and the expression of FSHR and actR genes was measured by SYBR-Green Real-Time PCR. GraphPad Prism was used for statistical analysis of data, and the t-Student's test was used to compare the results between the two groups. PCOS rats had longer and irregular estrous cycles compared to controls. The expression of FSHR and actR genes were significantly decreased in the rat model of PCOS compared to control rats. In PCOS rats, genes expression ratios for FSHR and actR were 0.91 ± 0.11 times (P = 0.008) and 0.42 ± 0.13 times (P = 0.048) less than controls, respectively.Reduced expression of the FSHR and actR genes in ovarian GCs may be one of the mechanisms mediating PCOS-related disorders, especially abnormal ovarian folliculogenesis and ovulation dysfunction, following exposure to androgens during fetal life.© 2022. The Author(s), under exclusive licence to Springer Nature B.V.
Ovarian mitochondrial dynamics and cell fate regulation in an androgen-induced rat model of polycystic ovarian syndrome
[J].In this study, we investigated in an androgenized rat model the involvement of autophagy and mitochondrial dynamics in granulosa cells in the pathogenesis of polycystic ovarian syndrome (PCOS) and its modulation by exogenous gonadotropin (eCG). We found 5α-dihydrotestosterone (DHT) treatment reduces ovarian length and weight with predominantly late antral and/or preovulatory stage follicles and no corpora lutea. DHT increased the population of large lysosomes (>50 micron) and macroautophagy, an event associated with granulosa cell apoptosis. Increased granulosa cell Dynamin Related Protein 1 (Drp1) content in the DHT group was accompanied by increased circular and constricted, but reduced rod-shaped, mitochondria. eCG eliminated all atypical follicles and increased the number of late antral and preovulatory follicles with less granulosa cell apoptosis. eCG-treated rats had a higher proportion of connected mitochondria, and in combination with DHT had a lower proportion of circular and constricted mitochondria than rats treated with DHT alone, suggesting that eCG induces mitochondrial fusion and attenuates fission in granulosa cells. In summary, we observed that DHT-induced up-regulation of Drp1 is associated with excessive mitochondrial fission, macroautophagy and apoptosis in granulosa cells at the antral stage of development in an androgenized rat model for PCOS, a response partially attenuated by exogenous gonadotropin.
高雄激素诱导小鼠颗粒细胞氧化应激与炎症的研究
[J].
氧化应激在女性生殖功能损伤中的研究进展
[J].
The role of oxidative stress in ovarian aging: a review
[J].Ovarian aging refers to the process by which ovarian function declines until eventual failure. The pathogenesis of ovarian aging is complex and diverse; oxidative stress (OS) is considered to be a key factor. This review focuses on the fact that OS status accelerates the ovarian aging process by promoting apoptosis, inflammation, mitochondrial damage, telomere shortening and biomacromolecular damage. Current evidence suggests that aging, smoking, high-sugar diets, pressure, superovulation, chemotherapeutic agents and industrial pollutants can be factors that accelerate ovarian aging by exacerbating OS status. In addition, we review the role of nuclear factor E2-related factor 2 (Nrf2), Sirtuin (Sirt), mitogen-activated protein kinase (MAPK), protein kinase B (AKT), Forkhead box O (FoxO) and Klotho signaling pathways during the process of ovarian aging. We also explore the role of antioxidant therapies such as melatonin, vitamins, stem cell therapies, antioxidant monomers and Traditional Chinese Medicine (TCM), and investigate the roles of these supplements with respect to the reduction of OS and the improvement of ovarian function. This review provides a rationale for antioxidant therapy to improve ovarian aging.© 2022. The Author(s).
Arachidonic Acid in Follicular Fluid of PCOS Induces Oxidative Stress in a Human Ovarian Granulosa Tumor Cell Line (KGN) and Upregulates GDF15 Expression as a Response
[J].
Mitochondrial and glucose metabolic dysfunctions in granulosa cells induce impaired oocytes of polycystic ovary syndrome through Sirtuin 3
[J].
Low grade chronic inflammation in women with polycystic ovarian syndrome
[J].
Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome
[J].
The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis
[J].
Adipose mesenchymal stem cell-derived exosomal microRNAs ameliorate polycystic ovary syndrome by protecting against metabolic disturbances
[J].
Resveratrol improves ovarian state by inhibiting apoptosis of granulosa cells
[J].
Curcumin Inhibits Hyperandrogen-Induced IRE1α-XBP1 Pathway Activation by Activating the PI3K/AKT Signaling in Ovarian Granulosa Cells of PCOS Model Rats
[J].
姜黄素可缓解多囊卵巢综合征大鼠卵巢炎症状态并改善其功能
[J].为探讨姜黄素对多囊卵巢综合征(polycystic ovarian syndrome,PCOS)大鼠模型血清性激素水平及组织中免疫因子IL-6、CRP、TNF-α表达的影响,将70只雌性SD大鼠随机分为正常组(10只)和造模组(60只)。造模成功后将其分为PCOS模型组、高、中、低药物治疗组,给予各组大鼠腹腔注射姜黄素,治疗结束后收集大鼠血清并分离卵巢进行检测,计算卵巢指数、大鼠体质量;采用血糖检测仪检测各组大鼠空腹血糖(fasting blood glucose, FBG)、空腹血清胰岛素(fasting serum insulin,FINS)和稳态模型胰岛素抵抗指数(homeostasis model assessment-insulin resistance index,HOMA-IR)水平;采用ELISA检测试剂盒检测血清性激素[卵泡刺激素( follicle-stimulating hormone,FSH)、雌二醇(estradiol,E2)、黄体生成素(luteinizing hormone,LH)、孕酮(progesterone,P)、睾酮(testosterone,T)]水平及TNF-α、IL-6、CRP水平;采用免疫组织化学法检测卵巢组织中TNF-α和IL-6的表达。血清及组织学检测结果显示,药物治疗组与PCOS组相比血清中性激素及FBG、FINS、HOMA-IR水平得到改善(P<0.05);TNF-α、IL-6、CRP等炎性因子水平显著减少(P<0.05);PCOS组卵泡和卵巢颗粒层中TNF-α及IL-6表达水平显著高于正常组(P<0.05),药物干预后表达量显著降低(P<0.05)。研究提示,姜黄素对PCOS的炎症改善作用与其抑制TNF-α、IL-6和CRP的表达有关,可能通过此途径改善性激素水平并调节血糖水平。
柚皮素下调RIP1-RIP3-MLKL信号通路抑制多囊卵巢综合征大鼠卵巢颗粒细胞凋亡
[J].
黄芩苷对多囊卵巢综合征大鼠颗粒细胞增殖和凋亡的影响
[J].
淫羊藿苷调节SDF-1/CXCR4信号通路对多囊卵巢综合征大鼠卵巢颗粒细胞凋亡的影响
[J].
槲皮素对多囊卵巢综合征大鼠卵巢颗粒细胞增殖与凋亡的影响研究
[J].
益母草碱调节RhoA/ROCK信号通路对多囊卵巢综合征大鼠卵巢颗粒细胞自噬和凋亡的影响
[J].
The role of serum inflammatory cytokines and berberine in the insulin signaling pathway among women with polycystic ovary syndrome
[J].
黄芪甲苷对肥胖型多囊卵巢综合征大鼠胰岛素抵抗及MAPK/ERK通路的影响
[J].
毛兰素对多囊卵巢综合征大鼠卵巢颗粒细胞凋亡的影响及机制
[J].