
国际妇产科学杂志 ›› 2023, Vol. 50 ›› Issue (1): 109-115.doi: 10.12280/gjfckx.20220804
收稿日期:2022-10-05
出版日期:2023-02-15
发布日期:2023-03-02
通讯作者:
匡洪影,E-mail:基金资助:
SUN Chang, PAN Zi-meng, ZHAO Shan-shan, LI Jing, KUANG Hong-ying△( )
)
Received:2022-10-05
Published:2023-02-15
Online:2023-03-02
Contact:
KUANG Hong-ying, E-mail: 摘要:
多囊卵巢综合征(polycystic ovary syndrome,PCOS)是引起育龄期女性生殖障碍和代谢异常的常见妇科疾病,临床表现具有异质性。下丘脑炎症是一种以小胶质细胞激活为主要病理改变的低度炎性病变,被认为是PCOS代谢、生殖及临床异质性的病理和生理基础。研究表明,下丘脑在调节并维持机体代谢稳态和生殖功能中发挥重要作用;通过下丘脑炎症的诱发途径,提出了碳水化合物和饱和脂肪酸的共同过量摄入是其主要诱因。此外,下丘脑炎症在PCOS相关代谢紊乱和生殖障碍的发生、发展中具有重要作用及其潜在诱发途径,提示下丘脑各神经元以及中枢与外周之间异常的信号转导是诱发PCOS相关病理改变的关键途径,这些途径有望为临床治疗PCOS提供新思路、新靶点。
孙畅, 潘紫萌, 赵珊珊, 李婧, 匡洪影. 下丘脑炎症在多囊卵巢综合征中的调节机制[J]. 国际妇产科学杂志, 2023, 50(1): 109-115.
SUN Chang, PAN Zi-meng, ZHAO Shan-shan, LI Jing, KUANG Hong-ying. Regulation Mechanism of Hypothalamic Inflammation in Polycystic Ovary Syndrome[J]. Journal of International Obstetrics and Gynecology, 2023, 50(1): 109-115.
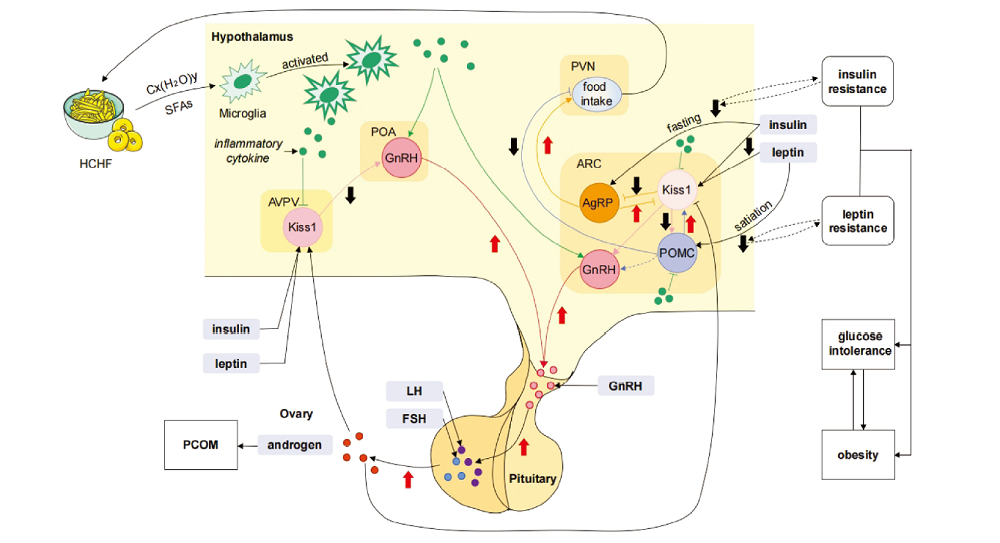
图1 下丘脑炎症参与调节PCOS相关代谢异常及生殖障碍机制图 注:HCHF 高碳水高脂肪饮食(high carbohydrate high fat diet),Cx(H2O)y 碳水化合物,SFAs饱和脂肪酸(saturated fatty acids),AVPV 前侧腹室周核(anteroventral periventricular nucleus),POA 视前区(preoptic area),PVN 室旁核(paraventricular nucleus),ARC 弓状核(arcuate nucleus),Kiss1 亲吻肽神经元1(kisspeptin neuron 1),GnRH 促性腺激素释放激素(gonadotropin releasing hormone),AgRP 刺鼠相关蛋白(agouti-related protein),POMC 阿黑皮素原神经元(proopiomelanocortin neuron),LH 黄体生成素(luteinizing hormone),FSH 卵泡刺激素(follicle-stimulating hormone)。
| [1] |
Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment[J]. Nat Rev Endocrinol, 2018, 14(5):270-284. doi: 10.1038/nrendo.2018.24.
doi: 10.1038/nrendo.2018.24 pmid: 29569621 |
| [2] |
Steegers-Theunissen R, Wiegel RE, Jansen PW, et al. Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence[J]. Int J Mol Sci, 2020, 21(21):8211. doi: 10.3390/ijms21218211.
doi: 10.3390/ijms21218211 |
| [3] |
Schneeberger M, Gomis R, Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance[J]. J Endocrinol, 2014, 220(2):T25-46. doi: 10.1530/JOE-13-0398.
doi: 10.1530/JOE-13-0398 |
| [4] |
Yoo ES, Yu J, Sohn JW. Neuroendocrine control of appetite and metabolism[J]. Exp Mol Med, 2021, 53(4):505-516. doi: 10.1038/s12276-021-00597-9.
doi: 10.1038/s12276-021-00597-9 |
| [5] |
Barlampa D, Bompoula MS, Bargiota A, et al. Hypothalamic Inflammation as a Potential Pathophysiologic Basis for the Heterogeneity of Clinical, Hormonal, and Metabolic Presentation in PCOS[J]. Nutrients, 2021, 13(2):520. doi: 10.3390/nu13020520.
doi: 10.3390/nu13020520 |
| [6] |
Dearden L, Buller S, Furigo IC, et al. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways[J]. Mol Metab, 2020, 42:101079. doi: 10.1016/j.molmet.2020.101079.
doi: 10.1016/j.molmet.2020.101079 |
| [7] |
Qin C, Li J, Tang K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases[J]. Endocrinology, 2018, 159(9):3458-3472. doi: 10.1210/en.2018-00453.
doi: 10.1210/en.2018-00453 pmid: 30052854 |
| [8] |
Cai M, Park HR, Yang EJ. Nutraceutical Interventions for Post-Traumatic Stress Disorder in Animal Models: A Focus on the Hypothalamic-Pituitary-Adrenal Axis[J]. Pharmaceuticals (Basel), 2022, 15(7):898. doi: 10.3390/ph15070898.
doi: 10.3390/ph15070898 |
| [9] |
Hirschberg PR, Sarkar P, Teegala SB, et al. Ventromedial hypothalamus glucose-inhibited neurones: A role in glucose and energy homeostasis?[J]. J Neuroendocrinol, 2020, 32(1):e12773. doi: 10.1111/jne.12773.
doi: 10.1111/jne.12773 |
| [10] |
Fosch A, Zagmutt S, Casals N, et al. New Insights of SF1 Neurons in Hypothalamic Regulation of Obesity and Diabetes[J]. Int J Mol Sci, 2021, 22(12):6186. doi: 10.3390/ijms22126186.
doi: 10.3390/ijms22126186 |
| [11] |
Gomez-Castro F, Zappettini S, Pressey JC, et al. Convergence of adenosine and GABA signaling for synapse stabilization during development[J]. Science, 2021, 374(6568): eabk2055. doi: 10.1126/science.abk2055.
doi: 10.1126/science.abk2055 |
| [12] |
Korf HW, Møller M. Arcuate nucleus, median eminence, and hypophysial pars tuberalis[J]. Handb Clin Neurol, 2021, 180:227-251. doi: 10.1016/B978-0-12-820107-7.00015-X.
doi: 10.1016/B978-0-12-820107-7.00015-X |
| [13] |
Roepke TA, Sadlier NC. REPRODUCTIVE TOXICOLOGY: Impact of endocrine disruptors on neurons expressing GnRH or kisspeptin and pituitary gonadotropins[J]. Reproduction, 2021, 162(5):F131-F145. doi: 10.1530/REP-20-0612.
doi: 10.1530/REP-20-0612 pmid: 34228631 |
| [14] |
Kung PH, Soriano-Mas C, Steward T. The influence of the subcortex and brain stem on overeating: How advances in functional neuroimaging can be applied to expand neurobiological models to beyond the cortex[J]. Rev Endocr Metab Disord, 2022, 23(4):719-731. doi: 10.1007/s11154-022-09720-1.
doi: 10.1007/s11154-022-09720-1 |
| [15] |
Arrigoni E, Chee M, Fuller PM. To eat or to sleep: That is a lateral hypothalamic question[J]. Neuropharmacology, 2019, 154:34-49. doi: 10.1016/j.neuropharm.2018.11.017.
doi: S0028-3908(18)30853-0 pmid: 30503993 |
| [16] |
Vandenbark AA, Offner H, Matejuk S, et al. Microglia and astrocyte involvement in neurodegeneration and brain cancer[J]. J Neuroinflammation, 2021, 18(1):298. doi: 10.1186/s12974-021-02355-0.
doi: 10.1186/s12974-021-02355-0 |
| [17] |
Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress[J]. J Neuroinflammation, 2021, 18(1):258. doi: 10.1186/s12974-021-02309-6.
doi: 10.1186/s12974-021-02309-6 |
| [18] |
Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans[J]. J Clin Invest, 2012, 122(1):153-162. doi: 10.1172/JCI59660.
doi: 10.1172/JCI59660 pmid: 22201683 |
| [19] |
André C, Guzman-Quevedo O, Rey C, et al. Inhibiting Microglia Expansion Prevents Diet-Induced Hypothalamic and Peripheral Inflammation[J]. Diabetes, 2017, 66(4):908-919. doi: 10.2337/db16-0586.
doi: 10.2337/db16-0586 pmid: 27903745 |
| [20] |
Lee I, Cooney LG, Saini S, et al. Increased odds of disordered eating in polycystic ovary syndrome: a systematic review and meta-analysis[J]. Eat Weight Disord, 2019, 24(5):787-797. doi: 10.1007/s40519-018-0533-y.
doi: 10.1007/s40519-018-0533-y |
| [21] |
Gao Y, Bielohuby M, Fleming T, et al. Dietary sugars, not lipids, drive hypothalamic inflammation[J]. Mol Metab, 2017, 6(8):897-908. doi: 10.1016/j.molmet.2017.06.008.
doi: S2212-8778(17)30239-9 pmid: 28752053 |
| [22] |
Sergi D, Boulestin H, Campbell FM, et al. The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction[J]. Mol Nutr Food Res, 2021, 65(1):e1900934. doi: 10.1002/mnfr.201900934.
doi: 10.1002/mnfr.201900934 |
| [23] |
Valdearcos M, Douglass JD, Robblee MM, et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility[J]. Cell Metab, 2018, 27(6):1356. doi: 10.1016/j.cmet.2018.04.019.
doi: S1550-4131(18)30303-6 pmid: 29874568 |
| [24] |
Benzler J, Ganjam GK, Pretz D, et al. Central inhibition of IKKβ/NF-κB signaling attenuates high-fat diet-induced obesity and glucose intolerance[J]. Diabetes, 2015, 64(6):2015-2027. doi: 10.2337/db14-0093.
doi: 10.2337/db14-0093 pmid: 25626735 |
| [25] |
Serna-Rodríguez MF, Bernal-Vega S, de la Barquera J, et al. The role of damage associated molecular pattern molecules (DAMPs) and permeability of the blood-brain barrier in depression and neuroinflammation[J]. J Neuroimmunol, 2022, 371:577951. doi: 10.1016/j.jneuroim.2022.577951.
doi: 10.1016/j.jneuroim.2022.577951 |
| [26] |
Cao Y, Li Z, Jiang W, et al. Reproductive functions of Kisspeptin/KISS1R Systems in the Periphery[J]. Reprod Biol Endocrinol, 2019, 17(1):65. doi: 10.1186/s12958-019-0511-x.
doi: 10.1186/s12958-019-0511-x |
| [27] |
Moura-Assis A, Nogueira P, de-Lima-Junior JC, et al. TLR4-interactor with leucine-rich repeats (TRIL) is involved in diet-induced hypothalamic inflammation[J]. Sci Rep, 2021, 11(1):18015. doi: 10.1038/s41598-021-97291-7.
doi: 10.1038/s41598-021-97291-7 pmid: 34504172 |
| [28] |
Navarro VM. Metabolic regulation of kisspeptin-the link between energy balance and reproduction[J]. Nat Rev Endocrinol, 2020, 16(8):407-420. doi: 10.1038/s41574-020-0363-7.
doi: 10.1038/s41574-020-0363-7 pmid: 32427949 |
| [29] |
Yuan C, Huang WQ, Guo JH, et al. Involvement of kisspeptin in androgen-induced hypothalamic endoplasmic reticulum stress and its rescuing effect in PCOS rats[J]. Biochim Biophys Acta Mol Basis Dis, 2021, 1867(12):166242. doi: 10.1016/j.bbadis.2021.166242.
doi: 10.1016/j.bbadis.2021.166242 |
| [30] |
Qiu J, Stincic TL, Bosch MA, et al. Deletion of Stim1 in Hypothalamic Arcuate Nucleus Kiss1 Neurons Potentiates Synchronous GCaMP Activity and Protects against Diet-Induced Obesity[J]. J Neurosci, 2021, 41(47):9688-9701. doi: 10.1523/JNEUROSCI.0622-21.2021.
doi: 10.1523/JNEUROSCI.0622-21.2021 pmid: 34654752 |
| [31] |
Könner AC, Klöckener T, Brüning JC. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond[J]. Physiol Behav, 2009, 97(5):632-638. doi: 10.1016/j.physbeh.2009.03.027.
doi: 10.1016/j.physbeh.2009.03.027 pmid: 19351541 |
| [32] |
Yan J, Zhang H, Yin Y, et al. Obesity-and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response[J]. Nat Med, 2014, 20(9):1001-1008. doi: 10.1038/nm.3616.
doi: 10.1038/nm.3616 |
| [33] |
Ullah R, Rauf N, Nabi G, et al. Mechanistic insight into high-fat diet-induced metabolic inflammation in the arcuate nucleus of the hypothalamus[J]. Biomed Pharmacother, 2021, 142:112012. doi: 10.1016/j.biopha.2021.112012.
doi: 10.1016/j.biopha.2021.112012 pmid: 34388531 |
| [34] |
Forrester SJ, Kikuchi DS, Hernandes MS, et al. Reactive Oxygen Species in Metabolic and Inflammatory Signaling[J]. Circ Res, 2018, 122(6):877-902. doi: 10.1161/CIRCRESAHA.117.311401.
doi: 10.1161/CIRCRESAHA.117.311401 pmid: 29700084 |
| [35] |
Chiurazzi M, Di Maro M, Cozzolino M, et al. Mitochondrial Dynamics and Microglia as New Targets in Metabolism Regulation[J]. Int J Mol Sci, 2020, 21(10):3450. doi: 10.3390/ijms21103450.
doi: 10.3390/ijms21103450 |
| [36] |
Fulton RE, Pearson-Smith JN, Huynh CQ, et al. Neuron-specific mitochondrial oxidative stress results in epilepsy, glucose dysregulation and a striking astrocyte response[J]. Neurobiol Dis, 2021, 158:105470. doi: 10.1016/j.nbd.2021.105470.
doi: 10.1016/j.nbd.2021.105470 |
| [37] |
Cunarro J, Casado S, Lugilde J, et al. Hypothalamic Mitochondrial Dysfunction as a Target in Obesity and Metabolic Disease[J]. Front Endocrinol (Lausanne), 2018, 9:283. doi: 10.3389/fendo.2018.00283.
doi: 10.3389/fendo.2018.00283 |
| [38] |
Kim JD, Yoon NA, Jin S, et al. Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding[J]. Cell Metab, 2019, 30(5):952-962.e5. doi: 10.1016/j.cmet.2019.08.010.
doi: S1550-4131(19)30439-5 pmid: 31495690 |
| [39] |
Desai M, Stiles L, Torsoni AS, et al. TNFα-Induced Oxidative Stress and Mitochondrial Dysfunction Alter Hypothalamic Neurogenesis and Promote Appetite Versus Satiety Neuropeptide Expression in Mice[J]. Brain Sci, 2022, 12(7):900. doi: 10.3390/brainsci12070900.
doi: 10.3390/brainsci12070900 |
| [40] |
Karbownik-Lewińska M, Stępniak J, Lewiński A. Potential Risk Factors for Isolated Hypothyroxinemia in Women of Childbearing Age-Results from Retrospective Analysis[J]. J Clin Med, 2021, 10(22):5384. doi: 10.3390/jcm10225384.
doi: 10.3390/jcm10225384 |
| [41] |
Schneider A, Saccon TD, Garcia DN, et al. The Interconnections Between Somatic and Ovarian Aging in Murine Models[J]. J Gerontol A Biol Sci Med Sci, 2021, 76(9):1579-1586. doi: 10.1093/gerona/glaa258.
doi: 10.1093/gerona/glaa258 |
| [42] |
Nestor CC, Qiu J, Padilla SL, et al. Optogenetic Stimulation of Arcuate Nucleus Kiss1 Neurons Reveals a Steroid-Dependent Glutamatergic Input to POMC and AgRP Neurons in Male Mice[J]. Mol Endocrinol, 2016, 30(6):630-644. doi: 10.1210/me.2016-1026.
doi: 10.1210/me.2016-1026 pmid: 27093227 |
| [43] |
Guzmán A, Hernández-Coronado CG, Rosales-Torres AM, et al. Leptin regulates neuropeptides associated with food intake and GnRH secretion[J]. Ann Endocrinol (Paris), 2019, 80(1):38-46. doi: 10.1016/j.ando.2018.07.012.
doi: S0003-4266(18)31212-5 pmid: 30243474 |
| [44] |
Valsamakis G, Lois K, Kumar S, et al. Metabolic and other effects of pioglitazone as an add-on therapy to metformin in the treatment of polycystic ovary syndrome(PCOS)[J]. Hormones (Athens), 2013, 12(3):363-378. doi: 10.1007/BF03401302.
doi: 10.1007/BF03401302 pmid: 24121378 |
| [45] |
Sergi D, Williams LM. Potential relationship between dietary long-chain saturated fatty acids and hypothalamic dysfunction in obesity[J]. Nutr Rev, 2020, 78(4):261-277. doi: 10.1093/nutrit/nuz056.
doi: 10.1093/nutrit/nuz056 pmid: 31532491 |
| [46] |
Lainez NM, Coss D. Obesity, Neuroinflammation, and Reproductive Function[J]. Endocrinology, 2019, 160(11):2719-2736. doi: 10.1210/en.2019-00487.
doi: 10.1210/en.2019-00487 pmid: 31513269 |
| [47] |
Counil H, Krantic S. Synaptic Activity and (Neuro)Inflammation in Alzheimer′s Disease: Could Exosomes be an Additional Link?[J]. J Alzheimers Dis, 2020, 74(4):1029-1043. doi: 10.3233/JAD-191237.
doi: 10.3233/JAD-191237 |
| [48] |
Passarelli A, Lettieri A, Nur Demirci T, et al. Gonadotropin-releasing hormone-secreting neuron development and function: an update[J]. Minerva Endocrinol (Torino), 2022, 47(1):58-69. doi: 10.23736/S2724-6507.22.03683-1.
doi: 10.23736/S2724-6507.22.03683-1 |
| [1] | 徐淑颖, 徐海鹏, 王丽娜, 张阳. 锌与多囊卵巢综合征的关系[J]. 国际妇产科学杂志, 2025, 52(2): 217-221. |
| [2] | 闫辉波, 张琳. 1990—2021年中国及全球多囊卵巢综合征疾病负担及预测分析[J]. 国际妇产科学杂志, 2025, 52(2): 228-233. |
| [3] | 陈淑琳, 乔峤. 阴道上皮损伤修复与微生态环境的关系[J]. 国际妇产科学杂志, 2025, 52(1): 52-56. |
| [4] | 张栋, 王筝, 李凯, 卞文丽, 高志华. 非妊娠期重度自发性卵巢过度刺激综合征一例[J]. 国际妇产科学杂志, 2025, 52(1): 79-83. |
| [5] | 胡蝶, 任佳杰, 刘佳宁, 冯晓玲. MAPK信号通路在PCOS中的作用机制及中药单体的药理研究进展[J]. 国际妇产科学杂志, 2024, 51(6): 684-691. |
| [6] | 李东楠, 向蓉, 汪海洋, 孙淼. 卵巢颗粒细胞凋亡在多囊卵巢综合征中的调控机制及中医药干预研究进展[J]. 国际妇产科学杂志, 2024, 51(6): 692-697. |
| [7] | 李晨曦, 范梦笑, 吴林玲, 窦真, 贾佳, 孙娅瑄. 高雄激素诱导多囊卵巢综合征神经内分泌紊乱的研究进展[J]. 国际妇产科学杂志, 2024, 51(6): 698-702. |
| [8] | 王雅慧, 王艳, 王艳, 裴飞. 胎儿生长受限的病因及对患儿远期健康的影响[J]. 国际妇产科学杂志, 2024, 51(2): 152-156. |
| [9] | 孙艺文, 熊可, 张梓絮, 翁雅婧, 王勇. 双酚A对多囊卵巢综合征发病机制的影响[J]. 国际妇产科学杂志, 2023, 50(6): 601-605. |
| [10] | 李振英, 孙晓彤, 邢广阳, 李晶晶, 柳婷婷, 张一凡. 鞘脂代谢与妇科良恶性疾病的研究进展[J]. 国际妇产科学杂志, 2023, 50(6): 649-654. |
| [11] | 黄晓彤, 程湘玮, 张阳. 子痫前期母胎界面TLR4作用的研究进展[J]. 国际妇产科学杂志, 2023, 50(6): 689-694. |
| [12] | 徐惠, 解秀珍. 多囊卵巢综合征患者负性情绪的研究进展[J]. 国际妇产科学杂志, 2023, 50(5): 530-534. |
| [13] | 寇丽辉, 宋殿荣, 郭洁. 负性情绪对多囊卵巢综合征不孕症的影响[J]. 国际妇产科学杂志, 2023, 50(5): 535-539. |
| [14] | 高雅, 鲁娣, 宋殿荣. 抗苗勒管激素在多囊卵巢综合征发病及诊治中的作用[J]. 国际妇产科学杂志, 2023, 50(5): 540-544. |
| [15] | 李晶晶, 孙晓彤, 柳婷婷, 李振英, 张一凡, 苏妍. 成纤维细胞生长因子21与妊娠期代谢性疾病的研究进展[J]. 国际妇产科学杂志, 2023, 50(4): 400-404. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||