
国际妇产科学杂志 ›› 2025, Vol. 52 ›› Issue (1): 88-93.doi: 10.12280/gjfckx.20240952
收稿日期:2024-10-22
出版日期:2025-02-15
发布日期:2025-02-14
通讯作者:
王永红,E-mail:wangyh19672000@126.com
作者简介:△审校者
基金资助:Received:2024-10-22
Published:2025-02-15
Online:2025-02-14
Contact:
WANG Yong-hong, E-mail: wangyh19672000@126.com
摘要:
子痫前期(preeclampsia,PE)是妊娠期特有的疾病,是孕产妇和围产儿发病和死亡的主要原因。目前PE的病因和发病机制尚未完全阐明,滋养细胞侵袭不足和子宫螺旋动脉重塑障碍是导致PE发病的重要因素。有研究表明,蜕膜自然杀伤(decidual natural killer,dNK)细胞具有参与子宫螺旋动脉重塑、调控滋养细胞入侵、参与母胎界面免疫耐受、促进胎儿生长等作用。dNK细胞数量、功能和表型等的变化,dNK细胞与绒毛外滋养细胞上配体结合的相应受体表达异常,dNK细胞分泌的细胞因子的变化,可能是PE发病的原因。综述dNK细胞在PE发生发展中的作用,为PE的诊断和治疗提供可靠的理论依据。
王晶, 王永红. 蜕膜自然杀伤细胞在子痫前期发病机制中的研究进展[J]. 国际妇产科学杂志, 2025, 52(1): 88-93.
WANG Jing, WANG Yong-hong. Decidual Natural Killer Cells in the Pathogenesis of Preeclampsia: A Review[J]. Journal of International Obstetrics and Gynecology, 2025, 52(1): 88-93.
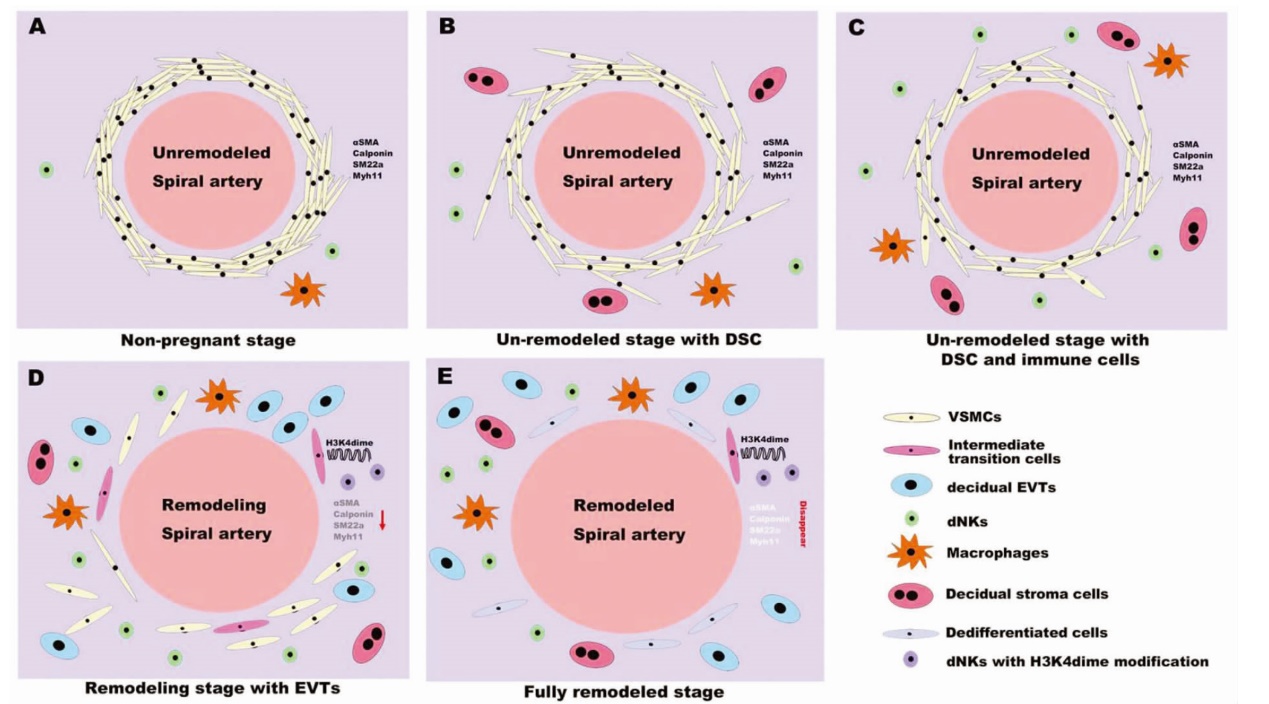
图1 子宫螺旋动脉重塑过程中VSMC的渐进转变[22] 注:A 在非妊娠期,VSMC在月经周期中保持不变,子宫内膜中存在少量NK细胞和巨噬细胞。B 子宫内膜间质细胞在胚胎植入后蜕膜化,并启动VSMC的形态转化。C dNK细胞和蜕膜巨噬细胞被募集到蜕膜,增强了VSMC的分离。D EVT侵入并与dNK细胞和蜕膜巨噬细胞协同作用,诱导VSMC去分化。重塑子宫螺旋动脉周围具有VSMC和dNK细胞双重特征的细胞(粉红色细胞)。E 子宫螺旋动脉周围VSMC消失。Unremodeled spiral artery 未重塑的子宫螺旋动脉;Non-pregnant stage 非妊娠期;Un-remodeled stage with DSC 蜕膜基质细胞出现的未重塑阶段;Un-remodeled stage with DSC and immune cells 蜕膜基质细胞和免疫细胞出现的未重塑阶段;Remodeling spiral artery 重塑过程中的子宫螺旋动脉;Remodeling stage with EVTs 绒毛外滋养细胞出现的重塑阶段;Remodeled spiral artery 重塑的子宫螺旋动脉;Fully remodeled stage 重塑完成阶段;Internmediate transition cells 中间过度细胞;decidual EVTs 蜕膜绒毛外滋养细胞;Macrophages 巨噬细胞;Decidual stromal cells 蜕膜基质细胞;Dedifferentiated cells 去分化细胞;dNKs with H3K4dime modification H3K4dime修饰的dNKs。
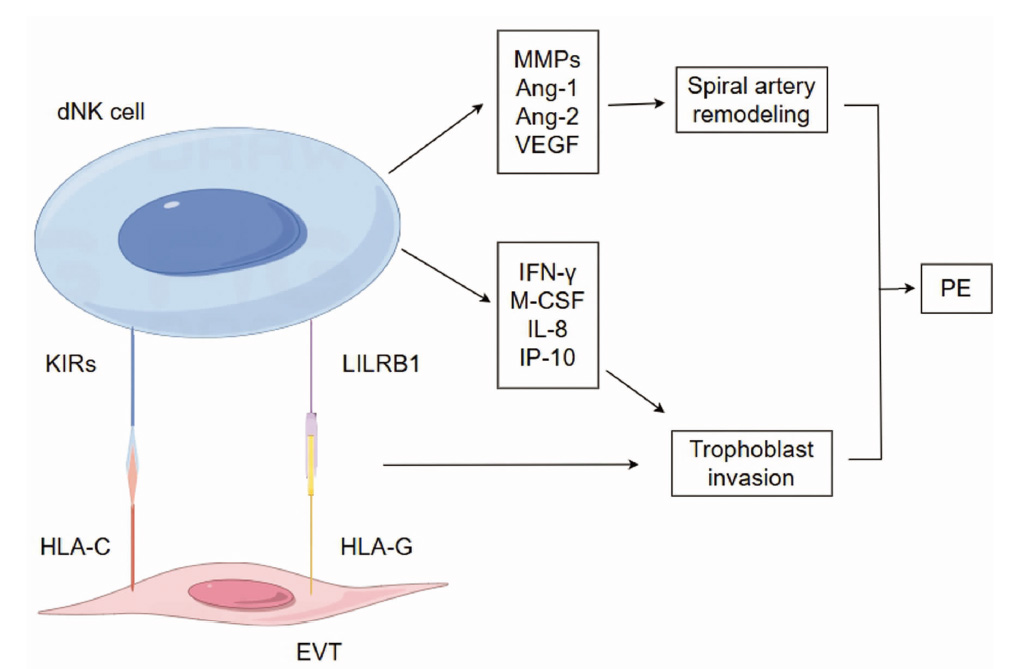
图2 dNK细胞在PE发病中的作用 注:KIRs 杀伤细胞免疫球蛋白样受体;LILRB1 白细胞免疫球蛋白样受体B1;MMPs 基质金属蛋白酶;Ang-1 血管生成素-1;Ang-2 血管生成素-2;VEGF 血管内皮生长因子;IFN-γ γ干扰素;M-CSF 巨噬细胞集落刺激因子;IP-10 IFN诱导蛋白-10;Spiral artery remodeling 子宫螺旋动脉重塑;Trophoblast invasion 滋养细胞侵袭。
| [1] | Rybak-Krzyszkowska M, Staniczek J, Kondracka A, et al. From Biomarkers to the Molecular Mechanism of Preeclampsia-A Comprehensive Literature Review[J]. Int J Mol Sci, 2023, 24(17):13252. doi: 10.3390/ijms241713252. |
| [2] |
Deer E, Herrock O, Campbell N, et al. The role of immune cells and mediators in preeclampsia[J]. Nat Rev Nephrol, 2023, 19(4):257-270. doi: 10.1038/s41581-022-00670-0.
pmid: 36635411 |
| [3] | Bisson C, Dautel S, Patel E, et al. Preeclampsia pathophysiology and adverse outcomes during pregnancy and postpartum[J]. Front Med (Lausanne), 2023,10:1144170. doi: 10.3389/fmed.2023.1144170. |
| [4] |
Ramdin S, Baijnath S, Naicker T, et al. The Clinical Value of Rodent Models in Understanding Preeclampsia Development and Progression[J]. Curr Hypertens Rep, 2023, 25(6):77-89. doi: 10.1007/s11906-023-01233-9.
pmid: 37043097 |
| [5] |
de Mendonça Vieira R, Meagher A, Crespo ÂC, et al. Human Term Pregnancy Decidual NK Cells Generate Distinct Cytotoxic Responses[J]. J Immunol, 2020, 204(12):3149-3159. doi: 10.4049/jimmunol.1901435.
pmid: 32376646 |
| [6] | Boulanger H, Bounan S, Mahdhi A, et al. Immunologic aspects of preeclampsia[J]. AJOG Glob Rep, 2024, 4(1):100321. doi: 10.1016/ j.xagr.2024.100321. |
| [7] |
Luo F, Yue J, Li L, et al. Narrative review of the relationship between the maternal-fetal interface immune tolerance and the onset of preeclampsia[J]. Ann Transl Med, 2022, 10(12):713. doi: 10.21037/atm-22-2287.
pmid: 35845477 |
| [8] | Yan S, Dong J, Qian C, et al. The mTORC1 Signaling Support Cellular Metabolism to Dictate Decidual NK Cells Function in Early Pregnancy[J]. Front Immunol, 2022,13:771732. doi: 10.3389/fimmu.2022.771732. |
| [9] | Wang P, Liang T, Zhan H, et al. Unique metabolism and protein expression signature in human decidual NK cells[J]. Front Immunol, 2023,14:1136652. doi: 10.3389/fimmu.2023.1136652. |
| [10] | Moffett A, Shreeve N. Local immune recognition of trophoblast in early human pregnancy: controversies and questions[J]. Nat Rev Immunol, 2023, 23(4):222-235. doi: 10.1038/s41577-022-00777-2. |
| [11] | Male V, Moffett A. Natural Killer Cells in the Human Uterine Mucosa[J]. Annu Rev Immunol, 2023,41:127-151. doi: 10.1146/annurev-immunol-102119-075119. |
| [12] |
Díaz-Hernández I, Alecsandru D, García-Velasco JA, et al. Uterine natural killer cells: from foe to friend in reproduction[J]. Hum Reprod Update, 2021, 27(4):720-746. doi: 10.1093/humupd/dmaa062.
pmid: 33528013 |
| [13] |
Wang F, Qualls AE, Marques-Fernandez L, et al. Biology and pathology of the uterine microenvironment and its natural killer cells[J]. Cell Mol Immunol, 2021, 18(9):2101-2113. doi: 10.1038/s41423-021-00739-z.
pmid: 34426671 |
| [14] | Strunz B, Bister J, Jönsson H, et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy[J]. Sci Immunol, 2021, 6(56):eabb7800. doi: 10.1126/sciimmunol.abb7800. |
| [15] | Lakatos KF, Hasselblatt K, Fülöp V, et al. Isolation and culture of decidual natural killer cells from term placenta and complete hydatidiform mole[J]. J Reprod Immunol, 2022,150:103475. doi: 10.1016/j.jri.2022.103475. |
| [16] | Luo F, Liu F, Guo Y, et al. Single-cell profiling reveals immune disturbances landscape and HLA-F-mediated immune tolerance at the maternal-fetal interface in preeclampsia[J]. Front Immunol, 2023,14:1234577. doi: 10.3389/fimmu.2023.1234577. |
| [17] |
Pengjie Z, Xionghui C, Yueming Z, et al. LncRNA uc003fir promotes CCL5 expression and negatively affects proliferation and migration of trophoblast cells in preeclampsia[J]. Pregnancy Hypertens, 2018, 14:90-96. doi: 10.1016/j.preghy.2018.08.449.
pmid: 30527126 |
| [18] | Zhang XN, Yang KD, Chen C, et al. Pericytes augment glioblastoma cell resistance to temozolomide through CCL5-CCR5 paracrine signaling[J]. Cell Res, 2021, 31(10):1072-1087. doi: 10.1038/s41422-021-00528-3. |
| [19] | Travis OK, Baik C, Tardo GA, et al. Adoptive transfer of placental ischemia-stimulated natural killer cells causes a preeclampsia-like phenotype in pregnant rats[J]. Am J Reprod Immunol, 2021, 85(6):e13386. doi: 10.1111/aji.13386. |
| [20] |
Varberg KM, Soares MJ. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling[J]. Placenta, 2021, 113:48-56. doi: 10.1016/j.placenta.2021.04.012.
pmid: 33985793 |
| [21] | Greenbaum S, Averbukh I, Soon E, et al. A spatially resolved timeline of the human maternal-fetal interface[J]. Nature, 2023, 619(7970):595-605. doi: 10.1038/s41586-023-06298-9. |
| [22] | Ma Y, Yu X, Zhang L, et al. Uterine decidual niche modulates the progressive dedifferentiation of spiral artery vascular smooth muscle cells during human pregnancy?[J]. Biol Reprod, 2021, 104(3):624-637. doi: 10.1093/biolre/ioaa208. |
| [23] | Robson A, Lash GE, Innes BA, et al. Uterine spiral artery muscle dedifferentiation[J]. Hum Reprod, 2019, 34(8):1428-1438. doi: 10.1093/humrep/dez124. |
| [24] | Hu J, Guo Q, Liu C, et al. Immune cell profiling of preeclamptic pregnant and postpartum women by single-cell RNA sequencing[J]. Int Rev Immunol, 2024, 43(1):1-12. doi: 10.1080/08830185.2022.2144291. |
| [25] | Du M, Wang W, Huang L, et al. Natural killer cells in the pathogenesis of preeclampsia: a double-edged sword[J]. J Matern Fetal Neonatal Med, 2022, 35(6):1028-1035. doi: 10.1080/14767058.2020.1740675. |
| [26] | Winship AL, Koga K, Menkhorst E, et al. Interleukin-11 alters placentation and causes preeclampsia features in mice[J]. Proc Natl Acad Sci U S A, 2015, 112(52):15928-15933. doi: 10.1073/pnas.1515076112. |
| [27] |
Han M, Hu L, Wu D, et al. IL-21R-STAT3 signalling initiates a differentiation program in uterine tissue-resident NK cells to support pregnancy[J]. Nat Commun, 2023, 14(1):7109. doi: 10.1038/s41467-023-42990-0.
pmid: 37925507 |
| [28] | Yang M, Wang M, Li N. Advances in pathogenesis of preeclampsia[J]. Arch Gynecol Obstet, 2024, 309(5):1815-1823. doi: 10.1007/s00404-024-07393-6. |
| [29] |
Park JY, Mani S, Clair G, et al. A microphysiological model of human trophoblast invasion during implantation[J]. Nat Commun, 2022, 13(1):1252. doi: 10.1038/s41467-022-28663-4.
pmid: 35292627 |
| [30] | Hackmon R, Pinnaduwage L, Zhang J, et al. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition[J]. Am J Reprod Immunol,2017 Jun, 77(6). doi: 10.1111/aji.12643. |
| [31] | Vento-Tormo R, Efremova M, Botting RA, et al. Single-cell reconstruction of the early maternal-fetal interface in humans[J]. Nature, 2018, 563(7731):347-353. doi: 10.1038/s41586-018-0698-6. |
| [32] | Wei X, Yang X. The novel role of activating receptor KIR2DS5 in preeclampsia[J]. Int Immunopharmacol, 2023, 125(Pt A):111087. doi: 10.1016/j.intimp.2023.111087. |
| [33] | Kelemu T, Erlandsson L, Seifu D, et al. Polymorphism in killer cell immunoglobulin-like receptors and human leukocyte antigen-c and predisposition to preeclampsia in Ethiopian pregnant women population[J]. J Reprod Immunol, 2020,141:103169. doi: 10.1016/j.jri.2020.103169. |
| [34] | Wedenoja S, Yoshihara M, Teder H, et al. Fetal HLA-G mediated immune tolerance and interferon response in preeclampsia[J]. EBioMedicine, 2020,59:102872. doi: 10.1016/j.ebiom.2020.102872. |
| [35] |
Zhang J, Dunk CE, Shynlova O, et al. TGFb1 suppresses the activation of distinct dNK subpopulations in preeclampsia[J]. EBioMedicine, 2019, 39:531-539. doi: 10.1016/j.ebiom.2018.12.015.
pmid: 30579870 |
| [36] | Fu YY, Ren CE, Qiao PY, et al. Uterine natural killer cells and recurrent spontaneous abortion[J]. Am J Reprod Immunol, 2021, 86(2):e13433. doi: 10.1111/aji.13433. |
| [37] |
Rytkönen KT, Adossa N, Zúñiga Norman S, et al. Gene Regulatory Network Analysis of Decidual Stromal Cells and Natural Killer Cells[J]. Reprod Sci, 2024, 31(10):3159-3174. doi: 10.1007/s43032-024-01653-1.
pmid: 39090334 |
| [38] | 赵钰林, 王永红. 核因子κB在子痫前期发病机制中的研究进展[J]. 国际妇产科学杂志, 2022, 49(1):56-59. doi: 10.12280/gjfckx.20210595. |
| [39] | Wang H, Liu ML, Chu C, et al. Paeonol alleviates placental inflammation and apoptosis in preeclampsia by inhibiting the JAK2/STAT3 signaling pathway[J]. Kaohsiung J Med Sci, 2022, 38(11):1103-1112. doi: 10.1002/kjm2.12585. |
| [40] | Jia W, Ma L, Yu X, et al. Human CD56+CD39+dNK cells support fetal survival through controlling trophoblastic cell fate: immune mechanisms of recurrent early pregnancy loss[J]. Natl Sci Rev, 2024, 11(6):nwae142. doi: 10.1093/nsr/nwae142. |
| [41] | Mahajan D, Sharma NR, Kancharla S, et al. Role of Natural Killer Cells during Pregnancy and Related Complications[J]. Biomolecules, 2022, 12(1):68. doi: 10.3390/biom12010068. |
| [42] |
Tavarna T, Wolfe B, Wu XJ, et al. Porphyromonas gingivalis-mediated disruption in spiral artery remodeling is associated with altered uterine NK cell populations and dysregulated IL-18 and Htra1[J]. Sci Rep, 2022, 12(1):14799. doi: 10.1038/s41598-022-19239-9.
pmid: 36042379 |
| [1] | 马玲, 李亚西, 赵敏, 王静, 李红丽. 细胞凋亡与不良妊娠结局关系的研究进展[J]. 国际妇产科学杂志, 2025, 52(2): 121-126. |
| [2] | 杨洋, 马媛, 陈宥艺, 赵静, 马文娟. 重度子痫前期患者血清外泌体对人正常蜕膜免疫细胞功能的影响[J]. 国际妇产科学杂志, 2025, 52(2): 143-152. |
| [3] | 石百超, 王宇, 常惠, 卢凤娟, 关木馨, 余健楠, 吴效科. 中药及天然产物改善子宫内膜异位症的作用机制[J]. 国际妇产科学杂志, 2025, 52(1): 66-71. |
| [4] | 张雯, 刘慧强. SOCS1与外泌体微小RNA在子痫前期发病机制中的作用[J]. 国际妇产科学杂志, 2025, 52(1): 94-98. |
| [5] | 王一丹, 王永红. 转化生长因子-β超家族在子痫前期发病机制中的作用[J]. 国际妇产科学杂志, 2025, 52(1): 99-104. |
| [6] | 樊博扬, 胡丽燕. 双胎妊娠合并子痫前期发病机制及预测方法研究进展[J]. 国际妇产科学杂志, 2024, 51(6): 611-615. |
| [7] | 邓玲玲, 伍绍文, 张为远. 小剂量阿司匹林在子痫前期预防中的研究进展[J]. 国际妇产科学杂志, 2024, 51(5): 515-518. |
| [8] | 张琦, 王新, 任毅, 刘超, 高慧婕. SLRPs在胎盘发育及妊娠相关疾病中的研究进展[J]. 国际妇产科学杂志, 2024, 51(5): 525-530. |
| [9] | 祝淡抹, 刘琴. 早产剖宫产后发现绒毛膜癌一例[J]. 国际妇产科学杂志, 2024, 51(5): 546-548. |
| [10] | 任毅, 胡玉莲, 王新, 张琦, 刘超, 高慧婕. 子痫前期的中药临床应用与现代药理学进展[J]. 国际妇产科学杂志, 2024, 51(4): 442-447. |
| [11] | 赵丽霞, 王小青. 硫酸镁在子痫前期治疗中的争议及其不良反应[J]. 国际妇产科学杂志, 2024, 51(4): 448-452. |
| [12] | 吴志韦, 林雪燕, 张雪芹, 杨梅琳. 子痫前期预防、预测的现状与关注焦点[J]. 国际妇产科学杂志, 2024, 51(3): 312-316. |
| [13] | 张春双, 董晓真, 周昌荣, 王懿珊, 栗河舟. 宫内治疗胎儿胸腔积液合并水肿并发镜像综合征一例[J]. 国际妇产科学杂志, 2024, 51(3): 357-360. |
| [14] | 彭兰, 柏婷, 周丽屏, 虞燕霞. 生物节律与子痫前期的相关性[J]. 国际妇产科学杂志, 2024, 51(2): 157-160. |
| [15] | 陈嘉颖, 冯亚玲. 蛋白质翻译后修饰在子痫前期发病机制中的研究进展[J]. 国际妇产科学杂志, 2024, 51(1): 1-4. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
